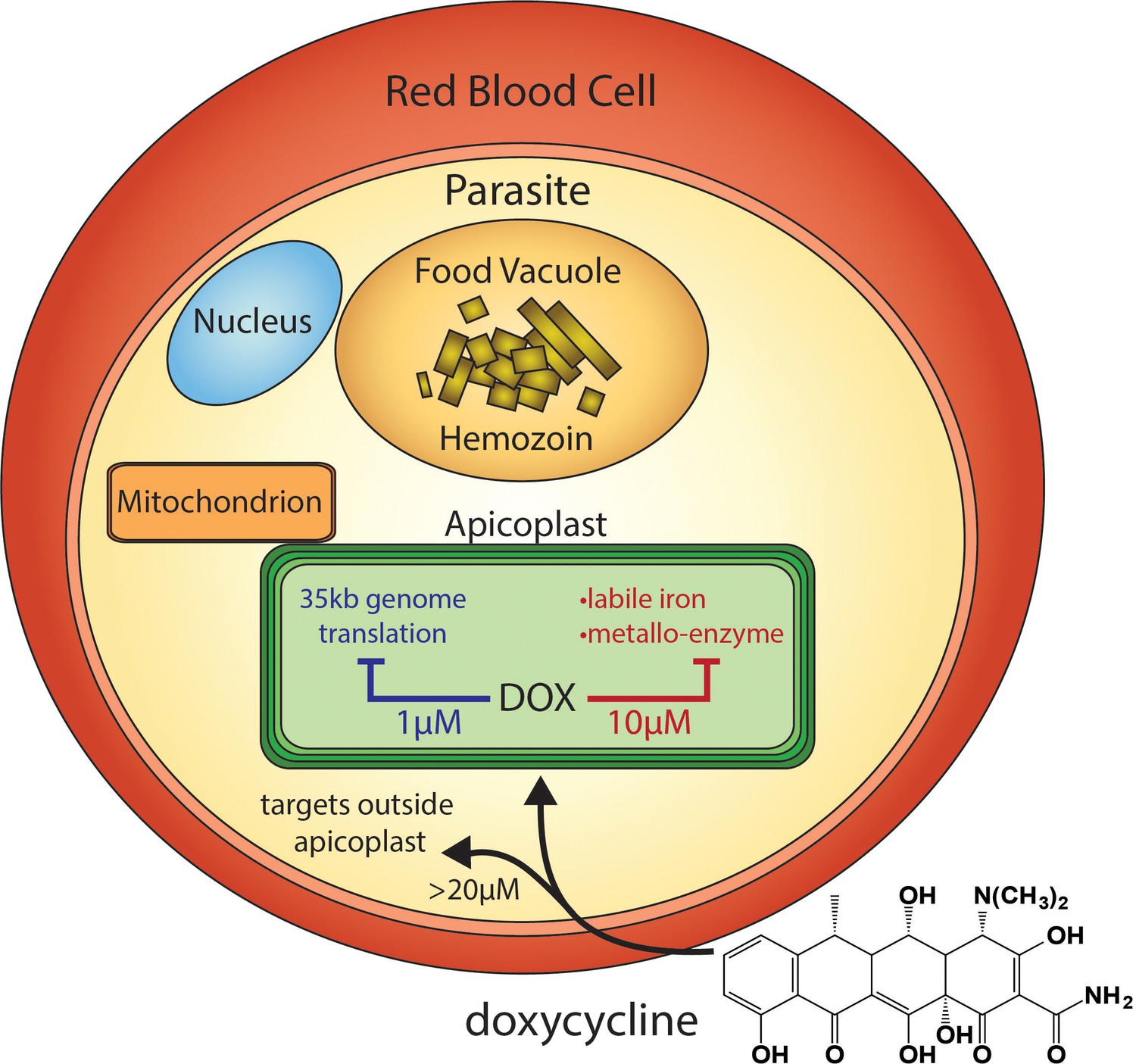
Doxycycline undergoes significant metabolism primarily in the liver, with only a small fraction excreted unchanged in the urine. As a tetracycline antibiotic, it is crucial to understand its pharmacokinetics to optimize therapeutic outcomes. The primary metabolic pathway involves conjugation, predominantly forming glucuronides, which account for increased solubility and easier excretion.
Absorption of doxycycline is nearly complete when taken orally, with peak plasma concentration achieved within 2 to 4 hours. Several factors influence its bioavailability, including the presence of food, which may decrease absorption. To maximize effectiveness, advise patients to take doxycycline on an empty stomach and with a full glass of water to reduce the risk of esophageal irritation.
Hepatic metabolism plays a pivotal role in the drug’s clearance. Enzymes like cytochrome P450 contribute to the biotransformation of doxycycline, though to a lesser extent than other antibiotics. Monitoring liver function may be necessary, especially in patients with pre-existing liver conditions or those on multiple medications that could interfere with liver metabolism.
Understanding these metabolic pathways not only aids in effective usage but also helps mitigate potential drug interactions. Clinicians should educate patients regarding the importance of adhering to prescribed dosages and timing to ensure optimal therapeutic levels of doxycycline in the body.
- Metabolism of Doxycycline
- Key Metabolic Pathways
- Factors Influencing Metabolism
- Pharmacokinetics of Doxycycline: Absorption and Distribution
- Factors Affecting Absorption
- Volume of Distribution
- Hepatic Metabolism Pathways of Doxycycline
- Elimination and Excretion Mechanisms of Doxycycline
- Impact of Drug Interactions on Doxycycline Metabolism
- CYP450 Enzyme Interaction
- Impact of Warfarin and Other Anticoagulants
Metabolism of Doxycycline
Doxycycline undergoes hepatic metabolism primarily through the cytochrome P450 enzyme system. About 40% of the drug is metabolized, while the remaining 60% is excreted unchanged in the urine. This metabolic process produces inactive metabolites that contribute to the drug’s pharmacokinetics.
Key Metabolic Pathways
The main pathways of doxycycline metabolism involve oxidation and conjugation. The primary enzymes implicated in doxycycline metabolism include CYP3A4 and other CYP450 isoforms. Understanding these pathways helps predict potential drug interactions, particularly with medications that induce or inhibit these enzymes.
Hydroxylation, a common phase I reaction, results in the formation of hydroxydoxycycline, which further undergoes conjugation. This increases water solubility, facilitating renal excretion.
Factors Influencing Metabolism
The metabolic rate of doxycycline is influenced by various factors, including age, liver function, and concurrent medications. Elderly patients may exhibit altered metabolism due to decreased liver function. Co-administration with drugs that affect hepatic enzymes can enhance or reduce doxycycline levels, impacting therapeutic efficacy.
Monitoring patients for potential side effects or decreased effectiveness is advisable when doxycycline is used alongside known enzyme modulators. This proactive approach ensures optimal treatment outcomes while minimizing risks.
For individuals with impaired liver function, dosage adjustments may be necessary to prevent toxicity. Regular assessment of liver enzymes can aid in guiding treatment and monitoring metabolism.
Pharmacokinetics of Doxycycline: Absorption and Distribution
Doxycycline displays rapid absorption following oral administration. Peak plasma concentrations occur approximately 2 to 4 hours after taking the medication. Food intake can influence absorption rates, leading to reduced bioavailability; thus, doxycycline is best taken on an empty stomach for optimal results. However, the presence of dairy products doesn’t significantly impact absorption.
Factors Affecting Absorption
- Gastrointestinal pH: Acidic conditions may hinder doxycycline solubility.
- Presence of divalent and trivalent metal ions: Calcium, magnesium, and iron can form chelates, decreasing absorption.
- Formulation: Immediate-release tablets have different absorption profiles compared to extended-release versions.
Once absorbed, doxycycline is widely distributed throughout the body. It penetrates various tissues, including lungs, liver, kidneys, and bone. This extensive distribution contributes to its effectiveness in treating a range of infections. High plasma protein binding (around 90-99%) ensures prolonged action in systemic circulation.
Volume of Distribution
The volume of distribution (Vd) for doxycycline averages between 0.7 to 1.5 L/kg. This indicates significant distribution beyond the vascular compartment, facilitating therapeutic effects in various tissues.
Renal excretion occurs primarily through the conjugated form, and certain metabolites can be eliminated through feces. Regular monitoring may be advisable, especially for patients with compromised renal function. Adjustments in dosage can optimize treatment outcomes while minimizing side effects.
Hepatic Metabolism Pathways of Doxycycline
Doxycycline primarily undergoes hepatic metabolism through several enzymatic pathways. The cytochrome P450 system plays a crucial role, with CYP3A4 being the most significant isoform involved in its biotransformation. This pathway leads to the formation of metabolites that are less active than the parent compound.
The metabolism begins with the oxidation of doxycycline, which may produce 5-hydroxy and 6-hydroxy doxycycline. These metabolites exhibit reduced antibacterial activity but can contribute to the drug’s overall disposition. Additionally, N-demethylation also occurs, leading to the production of smaller molecules that further progress through various conjugation reactions.
Glucuronidation and sulfation are key conjugation pathways for the metabolites. These reactions facilitate the excretion of doxycycline and its derivatives through the kidneys. Accurate monitoring of liver function is advisable for patients on long-term doxycycline therapy, as hepatic impairment can alter these metabolic pathways and affect drug levels in circulation.
Interindividual variations in metabolism can arise from genetic polymorphisms in cytochrome P450 enzymes, influencing treatment outcomes and side effects. It is beneficial to review an individual’s entire medication profile, as drugs that induce or inhibit CYP3A4 can significantly impact doxycycline metabolism, necessitating potential dosage adjustments.
In summary, understanding the hepatic metabolism of doxycycline fosters informed decision-making in clinical settings, ensuring optimal therapeutic efficacy while minimizing adverse effects.
Elimination and Excretion Mechanisms of Doxycycline
Doxycycline is primarily eliminated through hepatic metabolism and renal excretion. Approximately 40% of an administered dose is excreted unchanged in urine, while the liver processes the remaining portion into various metabolites.
In the liver, doxycycline undergoes biotransformation, mainly through enzyme-mediated processes such as conjugation. This metabolic pathway reduces its pharmacological activity and prepares it for excretion.
The renal clearance of doxycycline is significant; patients with compromised renal function require dosage adjustments. Monitoring renal function periodically is advisable for those on long-term doxycycline therapy to optimize drug levels and minimize toxicity.
Fecal elimination also plays a role, with about 60% of the drug appearing in bile and excreted in the stool. This route highlights the drug’s enterohepatic circulation, where it can be reabsorbed, affecting overall drug availability.
The half-life of doxycycline ranges from 18 to 22 hours in patients with normal renal function, providing guidelines for dosing intervals. Understanding these elimination and excretion dynamics is critical for effective treatment regimens.
Patients should be advised to stay hydrated to support renal function and facilitate the excretion process. Regular assessments of liver and kidney function ensure safety and efficacy during doxycycline therapy.
Impact of Drug Interactions on Doxycycline Metabolism
Drug interactions significantly influence the metabolism of doxycycline, altering its pharmacokinetics and clinical effectiveness. For instance, concurrent administration of doxycycline with antacids containing aluminum, magnesium, or calcium can inhibit its absorption. These metal ions bind effectively to doxycycline, forming insoluble complexes that diminish its bioavailability. It is advisable to maintain a gap of at least two to six hours between doxycycline administration and the use of such antacids to avoid this interaction.
CYP450 Enzyme Interaction
Certain medications can affect the cytochrome P450 enzyme system, which plays a role in the metabolism of doxycycline. Drugs like rifampicin enhance the metabolism of doxycycline by inducing CYP450 enzymes, potentially leading to decreased serum levels and reduced therapeutic efficacy. Monitoring patient response and adjusting doxycycline dosage may be necessary with such drug combinations.
Impact of Warfarin and Other Anticoagulants
Doxycycline may also interact with anticoagulants like warfarin. This combination can potentiate anticoagulant effects, increasing the risk of bleeding. Regular monitoring of INR levels is crucial when using doxycycline alongside warfarin, ensuring that doses are appropriately adjusted based on coagulation status.